Sciencemedicine
Type 2 diabetes physically changes the human heart, study finds
KE
3 days ago7 min read
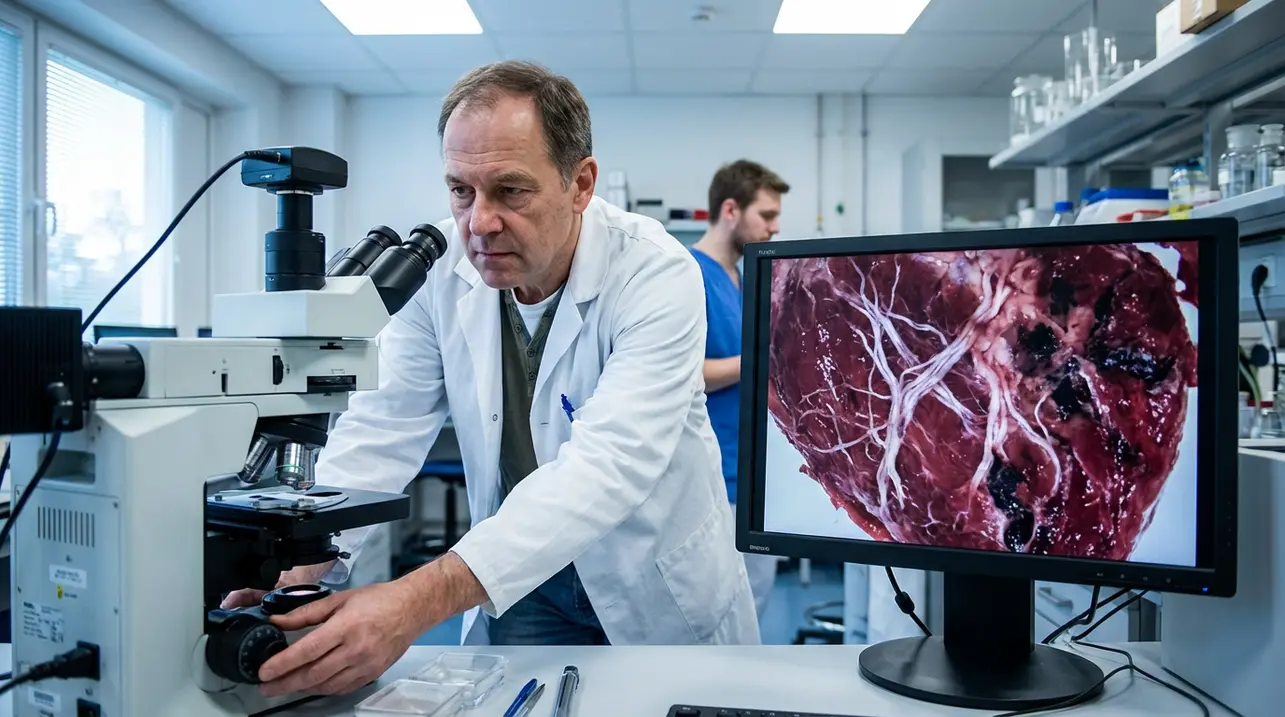
A landmark study has revealed that Type 2 diabetes does far more than simply elevate cardiovascular risk; it actively and physically remodels the very architecture of the human heart, a finding that fundamentally shifts our understanding of diabetic cardiomyopathy. Researchers conducting a meticulous analysis of donated human cardiac tissue discovered a trifecta of pathological alterations: a profound disruption in the cellular energy production mechanisms within heart muscle cells, a significant weakening of the muscle's structural integrity, and a dangerous accumulation of stiff, fibrous scar tissue that directly impairs the organ's pumping efficiency.These changes, the study notes, are not merely additive but synergistic, creating a perfect storm of cardiac dysfunction that is markedly exacerbated in individuals who also suffer from ischemic heart disease, the leading precursor to heart failure globally. This research, emerging from the intersection of cardiology, endocrinology, and molecular biology, provides a stark, cellular-level explanation for why heart failure remains the most common cause of death among the hundreds of millions living with Type 2 diabetes worldwide, moving beyond statistical correlation to demonstrate causative physical transformation.The energy crisis within the cardiomyocytes—the heart's workhorse cells—is particularly critical, as these cells have the highest mitochondrial density in the body to fuel their relentless contractions; diabetes appears to sabotage this power grid, leaving the muscle energetically bankrupt and unable to meet even basic demands. Concurrently, the infiltration of fibrotic tissue, akin to biological concrete being poured between the delicate muscle fibers, robs the heart of its essential elasticity, forcing it to work harder to achieve less blood flow, a process that mirrors the stiffening seen in advanced aging but accelerated on a diabetic timeline.Experts in metabolic cardiology suggest these findings underscore the urgent need to view diabetes management not just through the lens of glucose control but as a comprehensive cardiac protection strategy, potentially revolutionizing treatment protocols to include earlier and more aggressive use of cardioprotective medications like SGLT2 inhibitors and GLP-1 receptor agonists, which have shown benefits beyond sugar lowering. The historical precedent here is sobering; for decades, the diabetic heart was considered a victim of coronary artery disease, but this study solidifies the concept of a direct, independent myocardial toxicity from the diabetic metabolic milieu—hyperglycemia, insulin resistance, and lipotoxicity—acting as a constant insult.Looking forward, the implications are vast for the future of personalized medicine, pointing toward advanced cardiac imaging biomarkers to detect this remodeling non-invasively long before symptoms arise, and for next-generation therapies targeting mitochondrial biogenesis or specific fibrotic pathways. The research also casts a harsh light on global public health strategies, emphasizing that without decisive action to curb the diabetes pandemic, healthcare systems from the United States and the United Kingdom to India and China will face an insurmountable wave of heart failure cases, representing not just a clinical challenge but a profound socioeconomic burden. In essence, this study transforms the diabetic heart from a statistical risk into a vividly detailed anatomical and physiological reality, a sobering reminder that chronic metabolic conditions write their history directly onto our most vital organs.
#featured
#type 2 diabetes
#heart disease
#cardiac remodeling
#fibrosis
#ischemic heart disease
#heart failure
#medical research
Related News
Comments
Loading comments...
© 2025 Outpoll Service LTD. All rights reserved.