Scienceneuroscience
Strange microscopic structures found in Long COVID blood
KE
Kevin White
2 hours ago7 min read2 comments
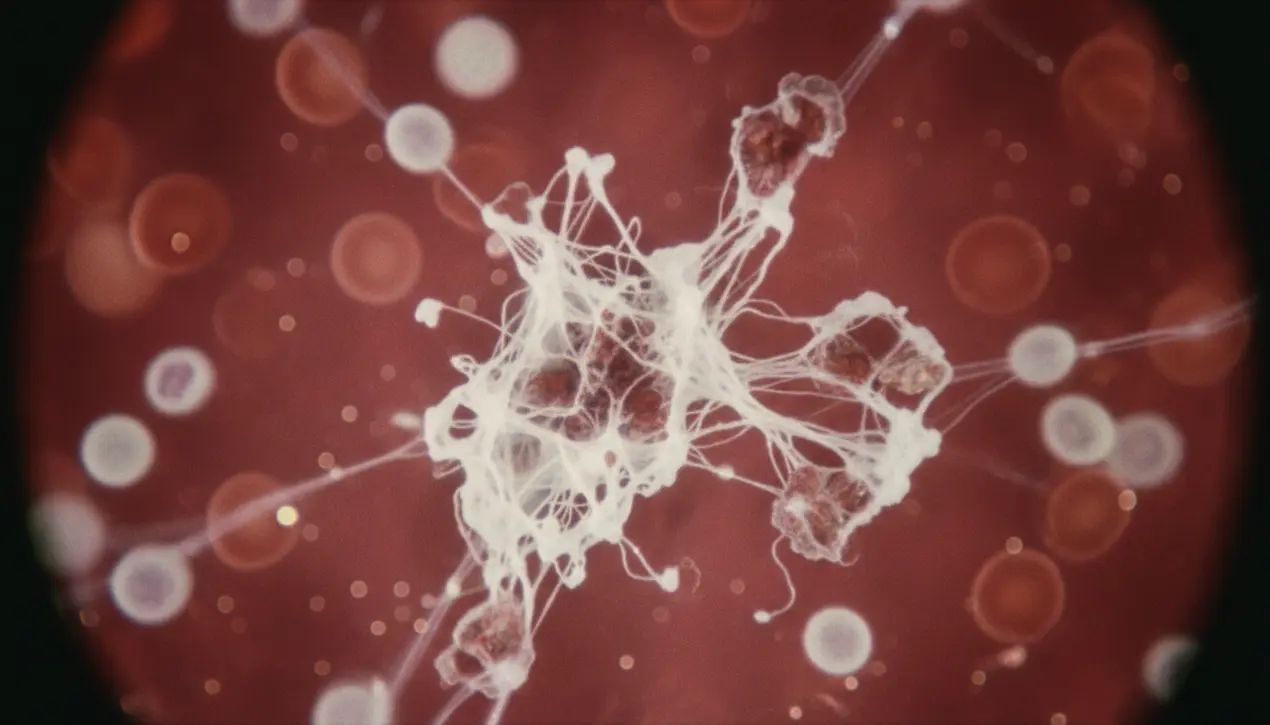
In a discovery that feels ripped from the pages of a near-future medical thriller, scientists have identified a bizarre and persistent biological signature in the blood of individuals suffering from Long COVID. These aren't your typical blood clots; they are intricate, microscopic structures best described as hybrid biological roadblocks.At their core are dense clusters of microclots, but they are inseparably tangled with what immunologists call neutrophil extracellular traps, or NETs. Think of NETs as a last-ditch, kamikaze defense mechanism where a type of white blood cell, the neutrophil, unravels its own DNA and laces it with toxic enzymes to create a sticky, web-like snare meant to capture pathogens.In the context of Long COVID, however, this defensive weapon appears to have gone haywire, creating a perfect storm with the microclots. The resulting composite structures are far more prevalent in Long COVID patients, and crucially, they are larger, denser, and exhibit a stubborn resilience not seen in the blood of healthy individuals, resisting the body's natural processes that would normally break them down.This finding, emerging from a confluence of advanced flow cytometry and proteomic analysis, is more than just a curious pathology; it represents a fundamental shift in our understanding of the post-viral condition. For the millions worldwide grappling with the debilitating fatigue, cognitive 'brain fog,' and exercise intolerance characteristic of Long COVID, this could be the elusive 'smoking gun.' These microscopic obstructions are theorized to be causing a capillary-level traffic jam throughout the body, severely impairing the delivery of oxygen and nutrients to tissues, from the brain to the muscles, effectively explaining the systemic nature of the symptoms. The implications for diagnostics are immediate and profound.Instead of relying on a constellation of subjective symptoms, clinicians may soon have a tangible, quantifiable biomarker—a way to objectively confirm a Long COVID diagnosis by simply analyzing a blood sample for the presence and density of these microclot-NET complexes. This moves the condition from a nebulous syndrome into the realm of a defined physiological disease.Therapeutically, this discovery opens up entirely new fronts in the battle against Long COVID. Current treatment is largely supportive and symptomatic, but now researchers can target the specific mechanisms that form these structures.This could involve repurposing existing fibrinolytic (clot-busting) drugs, developing novel therapies to inhibit the overzealous release of NETs, or a combination approach to dismantle these complexes from multiple angles. The work echoes historical precedents in medicine, such as the discovery of the Helicobacter pylori bacterium as the cause of peptic ulcers, which transformed a condition once attributed solely to stress into one curable with a simple course of antibiotics.While a single 'cure' for Long COVID remains on the horizon, this research provides a crucial, tangible target. It validates patients' experiences with hard biological data and charts a clear course for the next generation of biotech and pharmaceutical research, aiming not just to manage symptoms, but to dismantle the very architecture of the illness at a microscopic level.
#featured
#Long COVID
#microclots
#neutrophil extracellular traps
#blood analysis
#medical research
#post-viral illness
Stay Informed. Act Smarter.
Get weekly highlights, major headlines, and expert insights — then put your knowledge to work in our live prediction markets.
Related News
Comments
Loading comments...
© 2025 Outpoll Service LTD. All rights reserved.